Covid-19 Ag Rapid Test Kit
GenBody COVID-19 Ag is approved by the Australian Register of Therapeutic Goods (ARTG/TGA) for Supply in Australia*
The emergence of infective coronavirus (SARS-CoV-2) has generated significant global concern, especially with the disease (“COVID-19”) achieving pandemic status. While laboratory testing of nasopharyngeal swabs for active virus load represents the gold standard of infection testing and tracing, implementing on-site rapid testing for COVID-19 allows the potential for businesses to significantly reduce the waiting times associated with COVID-19 testing while also help maintain a COVID-safe workplace. In this light, Biogen Medi has partnered with GenBody and is now offering InnoScreen COVID-19 Antigen Rapid Test kits for sale to business clients.
Why should we conduct on-site rapid testing?
On-site screening may provide an immediate indication as to the possible infectivity of any individual on a given worksite, which will allow management to take swift and appropriate actions to maintain workplace safety. However, on-site rapid testing is not a substitute for maintaining good practice when it comes to infection control, COVID-safe plans and hygiene procedures should also be implemented.
Is the GenBody COVID-19 Ag TGA Approved?
Yes, it is. Find the GenBody COVID-19 Ag listed on the TGA site here.
What is included in each kit?
Each kit contains all the equipment required to perform 20 individual tests.
- Individually packed test device – 20
- Extraction tube with buffer – 20
- Sample collection swab – 20
- Tube stand – 1
- Instruction Guide – 1
Who should conduct testing using the GenBody COVID-19 Ag kits?
Tests must be conducted or supervised by a health practitioner as defined in Section 3 of the Therapeutic Goods Act 1989. Additionally, they must undergo training provided by Biogen Medi on correct use of the device and interpretation of the test result.
Will you supply training?
Yes. There is video, written and video conferencing support from our trained health practitioners.
How accurate are the results?
All results obtained from the GenBody COVID-19 Ag kits should be considered indicative only and should not be relied upon for diagnostic purposes. The manufacturer has stated a test specificity of 99.28% and an overall sensitivity rate of 96.00%. This means that there may be cases where a COVID-19-positive donor returns a negative test, particularly in the very early and late stages of infection due to the reduced viral load at these time points. For this reason, any symptomatic donors that return a negative test should be sent for immediate confirmation testing at a COVID-19 testing clinic and should not return to the workplace until a negative test result is obtained.
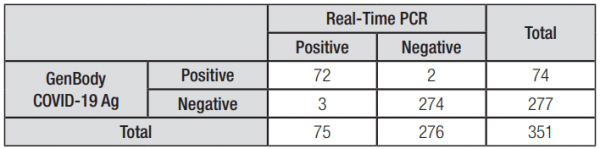
• Sensitivity = 96.0% (95% CI = 88.75% to 99.17%)
• Specificity = 99.28% (95% CI = 97.41% to 99.91%)
Who can purchase the test?
Leepac is able to sell to government, health practitioners and companies who employs or engages a health practitioner as defined in Section 3 of the Therapeutic Goods Act 1989 who is responsible for performing or supervising the performance of the test and undertakes suitable training on the testing device (training pack supplied by Biogen Medi).
Further clarification of these rules can be found on the TGA website here.
GenBody COVID-19 Ag is designed for the primary test of SARS-CoV-2 antigen and only provided for use by clinical laboratories or to healthcare workers for point-of-care testing, and not for at home testing.
* Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the virus. Follow-up testing with a molecular diagnostic should be considered to rule out infection in these individuals.
* Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1 and SARS-CoV
